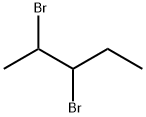
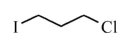
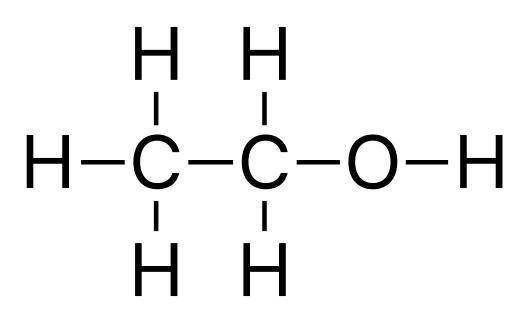
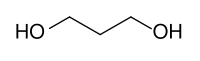
- carbon can form double & triple bonds with carbon atoms
- when multiple bonds form fewer hydrogen's are attached to the carbon atom
- naming rules are almost the same as with alkanes
-the position of the double/triple bonds always has the lowest number and is put in front of the parent chain
- double bonds(Alkenes) end in ENE
- triple bonds(Alkynes) end in YNE
Example:
Trans & Cis butene
- if two adjacent carbons are bonded by a double bond and have side chains on them two possible compounds are possible


Multiple double bonds
- more than one double bond can exist in a molecule
- use the same multipliers inside the parent chain
Example: